Investigation of the electrochemical behaviour of lead dioxide in aqueous sulfuric acid solutions by using the in situ EQCM technique
The paper authored by
Balázs Broda and
György Inzelt
is published in Journal of Solid State Electrochemistry (2020, vol. 24, pp. 1–10).
Abstract:
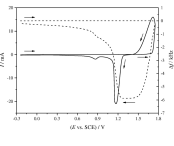
The charge-discharge characteristics and the aging mechanism of PbO2 layers in contact with sulfuric acid solutions of different concentrations (1.5–5.0 M) were studied by using combined cyclic voltammetry and electrochemical quartz crystal microbalance (EQCM) techniques. For this purpose, thick lead dioxide layers were electrodeposited on gold substrate from aqueous solutions of Pb(NO3)2 dissolved in nitric acid. Based on the electrochemical and the mass change responses, it was found that in more concentrated solutions of H2SO4, the main reduction reaction was the transformation of lead dioxide to lead sulfate. However, in less concentrated sulfuric acid media, the transformation of lead dioxide to lead(II) ion became the main reaction. These Pb2+ ions transformed into lead sulfate crystals later by a chemical reaction. Because the electrochemical oxidation of lead sulfate is less favourable in sulfuric acid medium of higher concentrations, thus, PbO2 layers cannot be tested by continuous cyclization, which is necessary to study their aging parameters. Therefore, a delay step before each cyclic voltammogram was applied while the non-conductive lead sulfate dissolves or alternatively, by applying a pre-oxidation step prior to each cyclic voltammetry experiment to produce electrochemically significant amount of lead dioxide which can be reduced during the following negative potential sweep.
